First published July 2, 2021
Post Edited September 20, 2022 to include updates.
At the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on October 22, 2020 Meeting, Steve Anderson, PhD, MPP
Director, Office of Biostatistics & Epidemiology, Center for Biologics Evaluation and Research (CBER) gave a presentation on CBER “Plans for Monitoring COVID-19 Vaccine Safety and Effectiveness”.
His presentation included a slide, below, about COVID-19 vaccine adverse event outcomes (injuries and deaths) which the FDA and CDC would be specifically monitoring. But he did not show the slide to VRBPAC, or the viewing public. He clicked right by it.
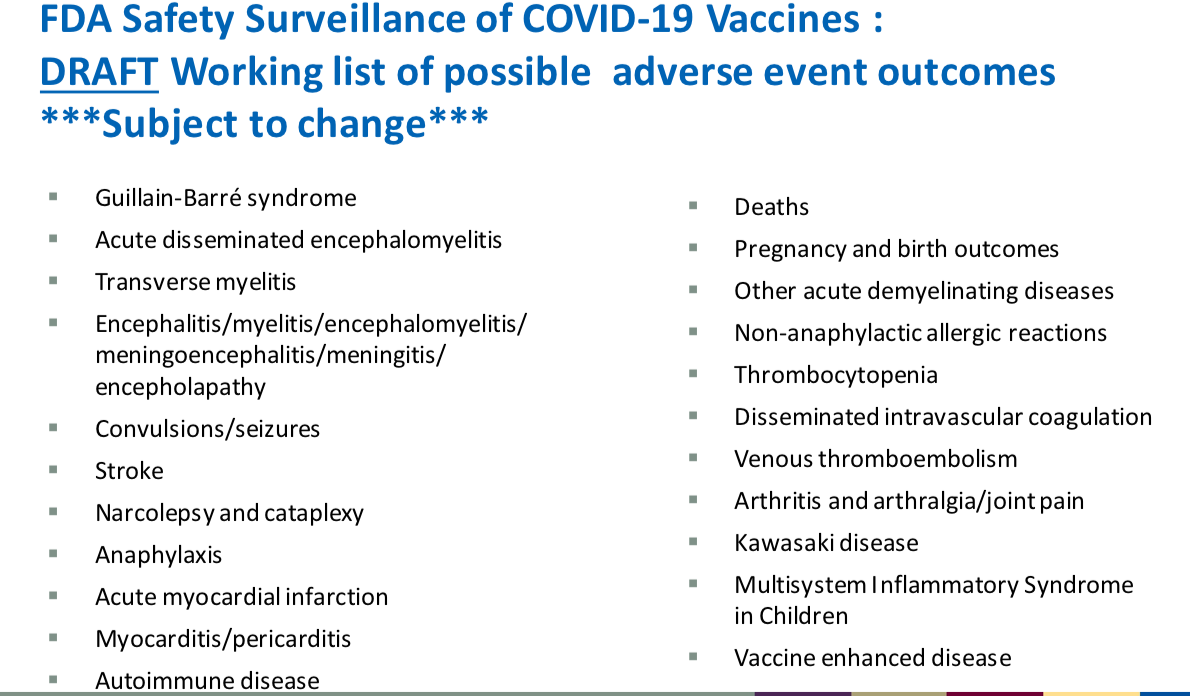
These side effect choices were not random. He explained they were based on evidence from the clinical trial data and from known science on the vaccine platform and components. This is from the transcript of the meeting.

Anderson stated in the above transcript that “we’ll be looking very closely at that data and especially the Phase 3 safety tads to identify potential safety questions . . .” However, since he made that statement, the FDA has allowed all of the COVID-19 vaccine makers to unblind their trials and offer shots to the control group, effectively ending the Phase 3 trials.
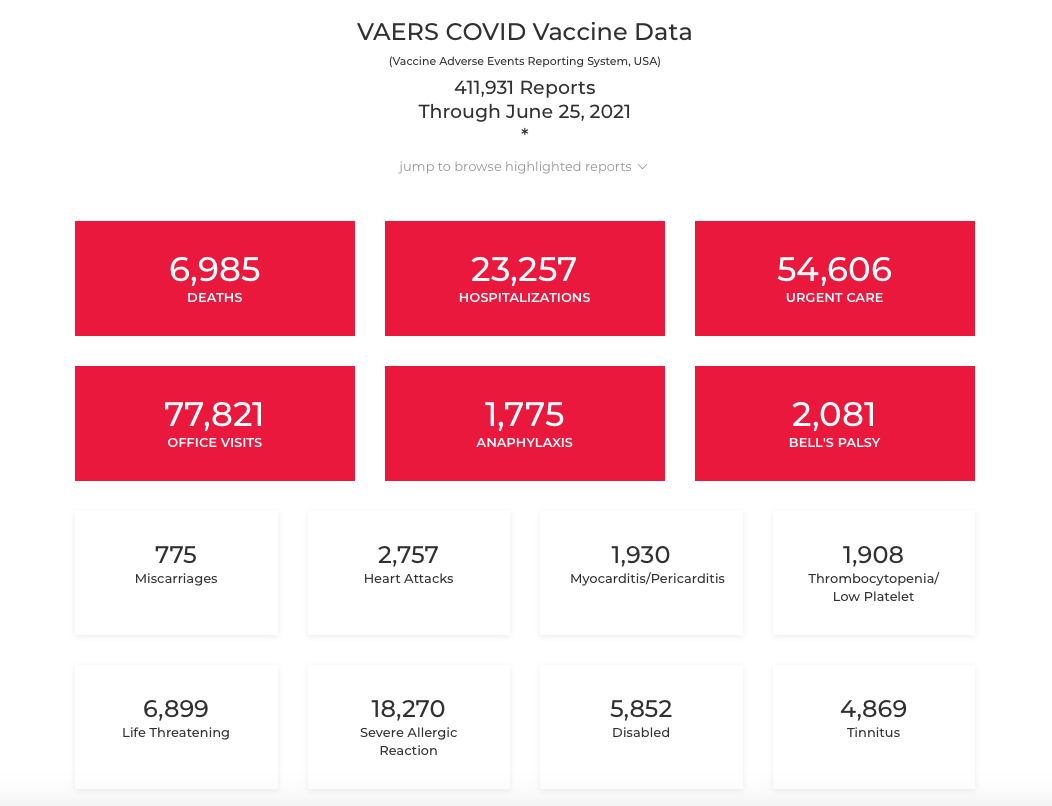
These vaccine adverse event outcomes are being reported to VAERS in unprecedented numbers. Here are the numbers from the date of our post in June 2021.
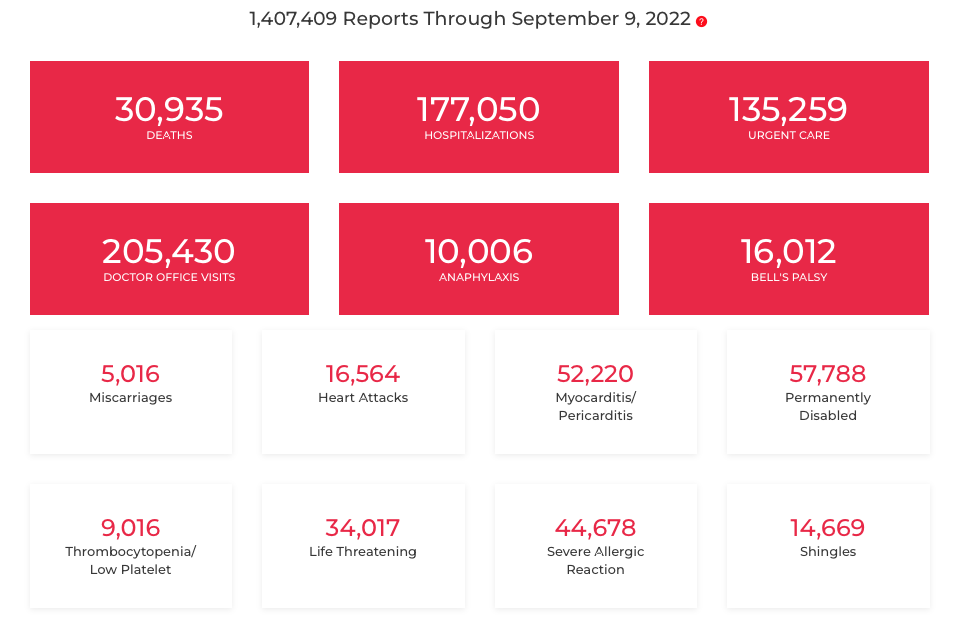
And here are the numbers as of September 9, 2022
In the 2020 VRBPAC presentation, Anderson says that “Tom” also has information about adverse outcomes. By “Tom” he means Tom Shimabukuro of the CDC, and his presentation started at about 1:59 in the video — before Anderson — but Shimabukuro didn’t talk about the information at all and he also clicked right through two slides without pausing. One was a list of vaccine adverse event outcomes they would be looking for in the passive Vaccine Adverse Event Reporting System (VAERS).

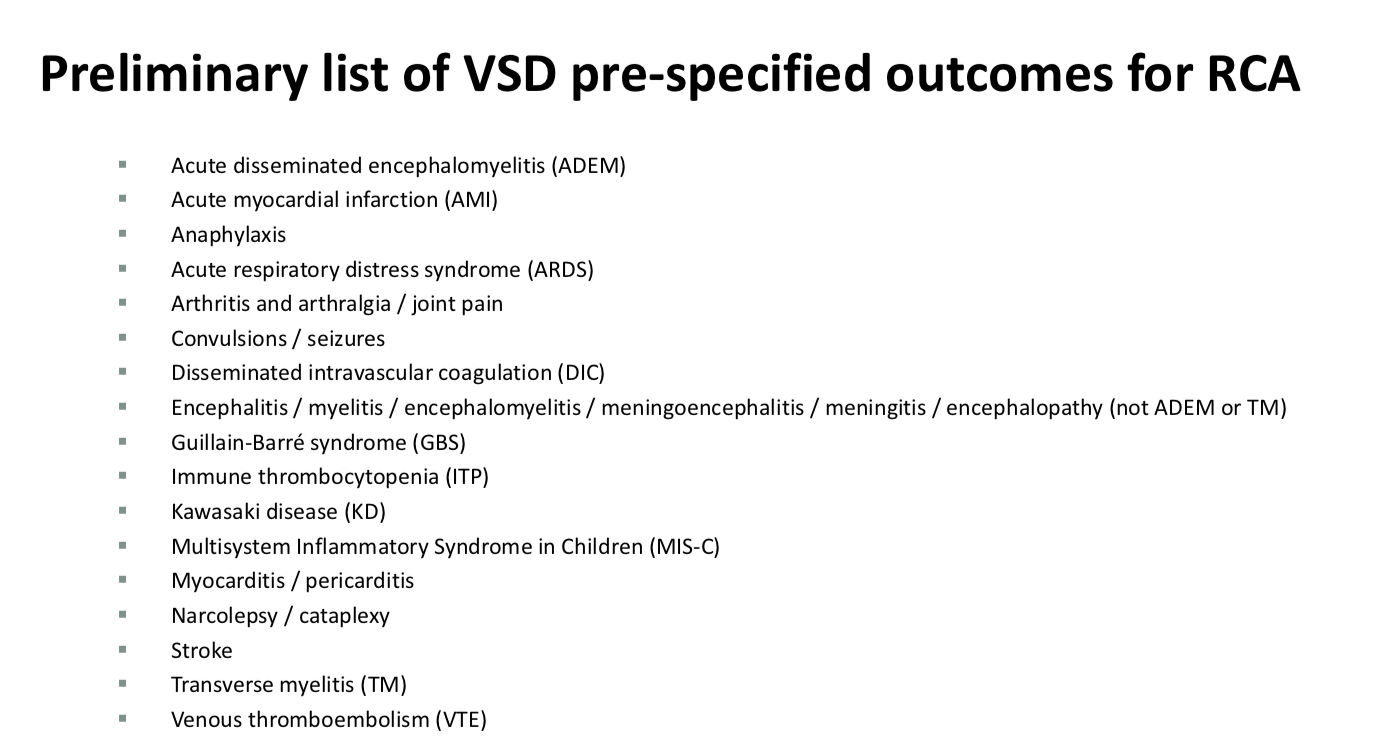
The other was a list of vaccine adverse event outcomes they would be looking for in CDC’s Vaccine Safety Datalink System (VSD). The public and most independent researchers have no access to this data for independent review.
The FDA has a new system launched in 2017 (a full decade after FDA Amendments Act of 2007 that required them to create an active postmarket risk and analysis system covering at least 100 million persons) called Biologics Effectiveness and Safety (BEST) System. The BEST system is now being used to try to establish background rates for the COVID-19 vaccine “Adverse Events of Special Interest” that the CDC and FDA will be monitoring with their systems.
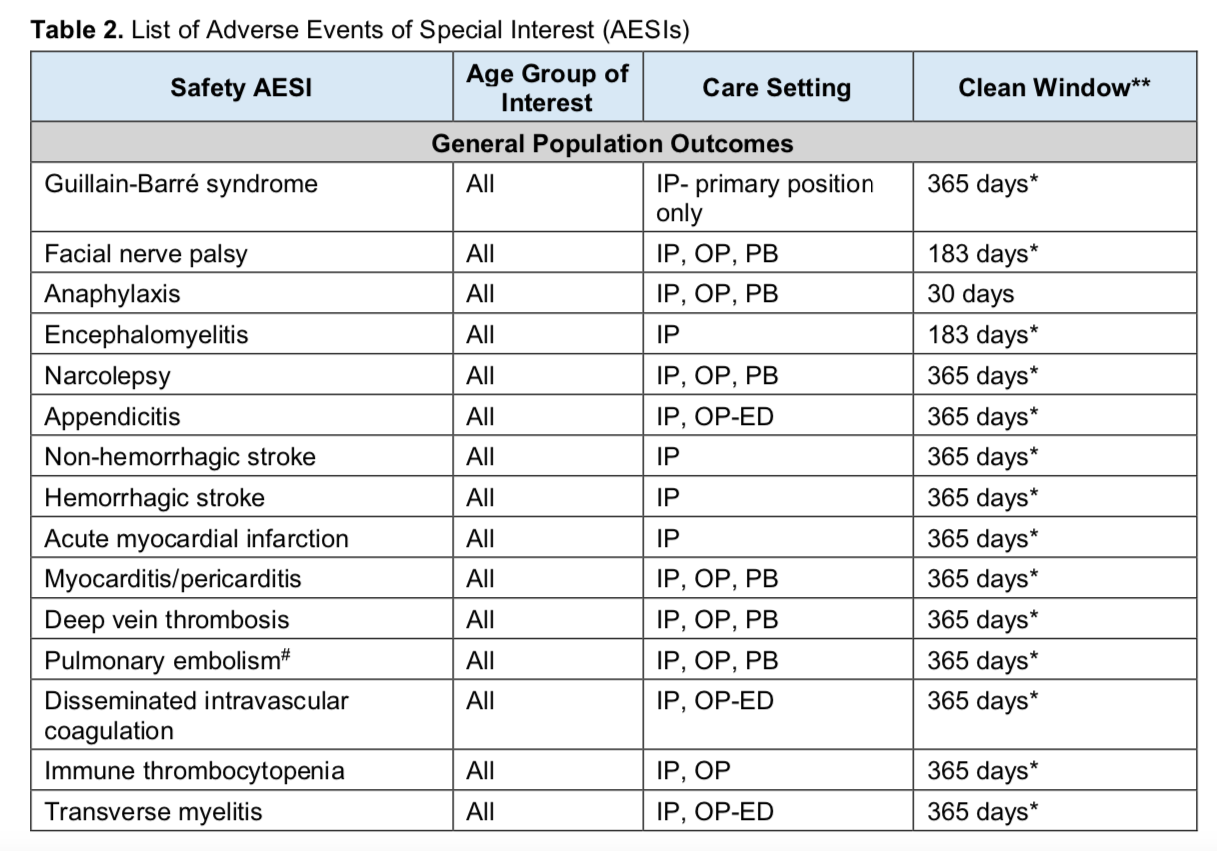
Given that global data analysis has shown a possible association between seasonal flu vaccination and COVID-19 disease severity, it’s interesting that the BEST study says:
To estimate incidence rates of AESIs in special populations of interest stratified by calendar year, sex, age group, and race/ethnicity (where reliably available) in each data source over the period 2017–2020. These populations will include:
o Older adults(i.e.,65 years old and abovea tcohort entry)
o Pediatric population(i.e.,0–17 years old at cohort entry)
o Pregnant women
o Individuals who received a seasonal influenza vaccine in the previous calendar year
During this COVID-19 crisis, both the FDA and CDC have made decisions that have not been in the best interest of the population or individuals. They have approved investigational products without sufficient safety or efficacy data, and they have actively censored or ignored existing treatments and natural immunity. They are actively partnering with the COVID-19 vaccine makers.
There are two important aspects of establishing whether reported adverse events are related to receipt of a vaccine. One is epidemiological. The rates of certain health issues in the general population are compared to the rates in people getting vaccinated. That’s what the FDA’s BEST study is about. Obviously, this information alone cannot rule causation in or out. Biological studies are also needed. Can the product cause the outcome seen? Ever since the 1986 National Childhood Vaccine Injury Act passed, removing liability from vaccine makers for injury or death for products recommended to children and pregnant women, the CDC has been in charge of vaccine safety and utterly failed in their duties. Biological studies are almost non-existent. The CDC prefers to use weaker epidemiological studies that are easily manipulated to desired outcome, to try to claim reported events are not associated.
Will they do the same for the COVID-19 vaccines? If so, will they get away with it? Since we first wrote that question, it has been answered. Yes, the federal oversight agencies are using contrived epidemiological studies, avoiding biological studies of their own while ignoring very concerning independent studies that are revealing the mechanisms of action that lead to harm. To find the latest, search Pubmed using keyword “COVID-19 vaccine” and an injury reported to VAERS, such as myocarditis, tinnitus, Guillain-Barre syndrome, Bell’s Palsy, etc.
Fortunately during COVID, researchers around the world have been awakened to the capture and corruption of public health agencies and they are beginning to do their own, independent studies. They are starting their own journals that have no ties to governments or the drug industry. A revolution is beginning within the ranks of doctors and scientists who believe in honest and ethical science and medicine.
Post References:
Tom Shimabukuro’s slides: https://www.fda.gov/media/143530/download
Steve Anderson’s slides: https://www.fda.gov/media/143557/download
Video of the meeting: https://youtu.be/1XTiL9rUpkg
Meeting transcript: https://www.fda.gov/media/143982/download
FDA page with links to all materials: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-october-22-2020-meeting-announcement#event-materials
BEST Background Rate study: https://www.bestinitiative.org/wp-content/uploads/2021/01/C19-Vaccine-Safety-AESI-Background-Rate-Protocol-2020.pdf

