Further Anomalies of the Oxford Coronavirus Vaccine
by John Stone, Guest Contributor
|Permalink| Republished here with permission.
On 27 April a New York Times article reported excitedly the result animal trials of the Oxford Coronavirus vaccine:
“Scientists at the National Institutes of Health’s Rocky Mountain Laboratory in Montana last month inoculated six rhesus macaque monkeys with single doses of the Oxford vaccine. The animals were then exposed to heavy quantities of the virus that is causing the pandemic… But more than 28 days later all six were healthy, said Vincent Munster, the researcher who conducted the test . . .”
This would have been just as well because just four days earlier on 23 April Oxford Vaccine Group under the leadership of Andrew Pollard amid immense publicity had begun experimenting on human subjects. On 30 April a contract was announced with AstraZeneca to manufacture the vaccine, promising to deliver an entirely new vaccine to the market at unprecedented speed by September. The only trouble was that when the results of the animal trial came to light in mid-May it was disclosed that on the contrary all the monkeys had become ill. The Daily Mail reported:
“In the latest animal trials of the vaccine carried out on rhesus macaques, all six of the participating monkeys went on to catch the coronavirus.
“Dr William Haseltine, a former Harvard Medical School professor, revealed the monkeys who received the vaccine had the same amount of virus in their noses as the three non-vaccinated monkeys in the trial.
This suggests the treatment, which has already received in the region of £90 million in government investment, may not halt the spread of the deadly disease.”
Haseltine also commented in Forbes:
“There is a second troubling result of the Oxford paper. The titer of neutralizing antibody, as judged by inhibition of virus replication by successive serum dilutions as reported is extremely low. Typically, neutralizing antibodies in effective vaccines can be diluted by more than a thousand fold and retain activity. In these experiments the serum could be diluted only by 4 to 40 fold before neutralizing activity was lost.”
Manifestly, human testing proceeded both against an entirely misleading background, and prematurely – which poses the most serious ethical questions. And now that we know that though the product was defective everything ploughs on regardless – Oxford/AstraZeneca now have contracts for hundreds of millions of rounds of the vaccine from both the British and the United States government. The British government has both a huge financial investment in the product and a reputational one, but it may help that Prof Pollard is both an adviser to the British regulator and chair of the committee recommends vaccine for public use.
John Stone is UK Editor for Age of Autism.
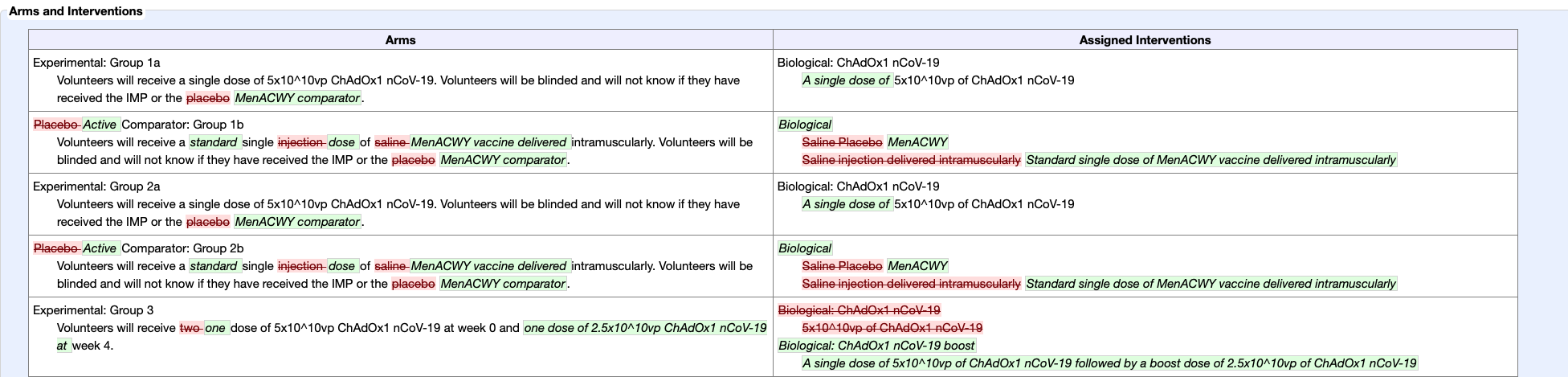
ICWA NOTE regarding changes to the human clinical trial protocol
It is also concerning that the human clinical trial design, now underway, was changed after filing. While this does sometimes happen, this change significantly changes the potential usefulness of the data derived. The use of an inert placebo was removed. In its place, control groups were given a dose of MenACWY vaccine. This change, will make the study results meaningless.
AstraZeneca has an absurd excuse for using a meningococcal vaccine in the control groups rather than an inert placebo in Phase II/III trials. FierceBiotech reports they are saying:
“The use of an active vaccine as a control is intended to ensure participants are unable to tell whether they received AZD1222 based on side effects such as soreness at the injection site. In the absence of such effects across both groups, participants could determine whether they had received the vaccine and make behavioral changes that skew the results of the study.”
This is ridiculous, highly unethical, and not the scientific method. The CDC pink book shows concerning risks of adverse reactions to MenACWY vaccines, including death.
A 1998 article in the BMJ states (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1113953/):
“In clinical settings where no gold standard treatment exists and where event rates vary widely, trial designs without placebo controls are unlikely to yield sensible result
AND
The ethics of recruiting patients into trials that cannot yield sensible results is dubious.”
If the COVID-19 vaccine group and the MenACWY vaccine group experience the same rate of “Headache, vasovagal syncope, dizziness, paresthesia, Guillain-Barré syndrome” — which are postmarking adverse events reported following one MenACWY vaccine product, will Astrazeneca simply say that those who received their COVID-19 vaccine had the same reactions as their control group? Deeming it “safe”? Because that’s exactly what all vaccine makers have been doing for the past few decades with nearly all other vaccines, in a pyramid scheme of comparisons that has no gold-standard inert placebo trials at its base.
In another article, the reason given to switch to an active control was
” . . . .because we expect to see some minor side effects from the ChAdOx1 nCOV-19 vaccine such as a sore arm, headache and fever. Saline does not cause any of these side effects. If participants were to receive only this vaccine or a saline control, and went on to develop side effects, they would be aware that they had received the new vaccine. It is critical for this study that participants remain blinded to whether or not they have received the vaccine, as, if they knew, this could affect their health behaviour in the community following vaccination, and may lead to a bias in the results of the study.”
But is it standard procedure to influence reactions by warning that it is expected the experimental vaccine would cause fever and headache? Thereby ensuring the participants are “unblinded” as soon as they DON’T experience those symptoms? Surely this is not the first clinical trial in which symptoms are expected in the experimental arm but not in the control arm. Actually, that is the POINT of inert placebos in any clinical trial, isn’t it? So that problems with the experimental product can be discovered? And the goal is to create a product that produces no side effects?
And it should be noted that while not as frequent as seen in vaccine recipients, some who receive saline injections DO experience pain and soreness at the injection site, and sometimes other symptoms are reported as well, such a headache, dizziness, and nausea. A well-designed and well-run ethical clinical trial would not jeopardize the validity of study results by eliminating an inert saline placebo arm.