YOUR ACTION NEEDED TODAY!
It takes just a few minutes to make your comment. Deadline is October 25th!
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will be holding a meeting to discuss a request to amend Pfizer-BioNTech’s Emergency Use Authorization (EUA) for administration of their COVID-19 mRNA vaccine to children 5 through 11 years of age.
Date: October 26, 2021
Time: 8:30 AM – 5:00 PM ET
The online web conference meeting will be available at the following:
Youtube: https://youtu.be/laaL0_xKmmAExternal
DEADLINE TO SUBMIT YOUR COMMENT IS OCTOBER 25. Submit your comments now!
Let your voice be heard and seen.
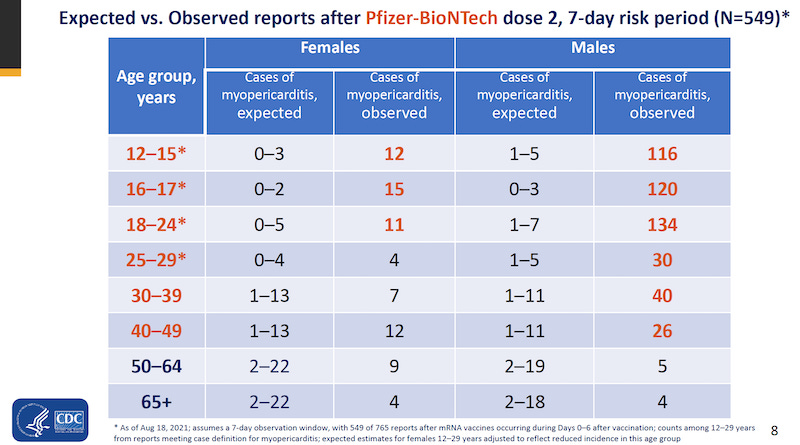
“The Pfizer mRNA vaccine causes catastrophic side effects, particularly heart inflammation (myocarditis and pericarditis) in youth. The CDC’s own analysis of “Myopericarditis following COVID-19 vaccination: Updates from the Vaccine Adverse Event Reporting System (VAERS)” showed astonishing increases particularly in children ages 12-15, 16-17, and 18-24.” From THIS post by Toby Rogers. Read the post for more information and action suggestions.
Dr. Bryan Ardis recorded a brief video showing how easy it is to post a comment at the Federal Register about your concerns for COVID-19 shots for children.
SUBMIT YOUR COMMENT NOW
