As Pfizer’s and Moderna’s investigational COVID-19 vaccines are being rolled out, we continue to see life-threatening anaphylactic reactions and deaths that follow at an alarming rate never seen with any other vaccine. Explore the Vaccine Adverse Event Reporting System Data.
The CDC has stated they suspect polyethylene glycol (PEG) in lipid nanoparticles (LNPs) that encapsulate mRNA vaccines to be the culprit for observed anaphylactic reactions in susceptible individuals. However, PEG allergy is relatively rare and this alone cannot explain the whole picture, as anaphylaxis has been occurring at a much higher than one-in-a-million expected frequency in mRNA vaccine recipients, even without any prior history of anaphylaxis.
Two Types of Anaphylaxis
1. IgE-mediated hypersensitivity to PEG and its cross-reactivity with polysorbates
Even though the majority of Americans (72%) have IgM and IgG antibodies to PEG—likely due to very frequent exposures to PEG as an inactive ingredient in common medications, processed foods, and skincare products—these antibody isotypes do not predispose to anaphylaxis. The presence of anti-PEG IgE might indeed be very rare, as anaphylaxis to PEG-containing medications has been reported to the FDA for a total of 53 cases, according to a case study published in 2019. Thus, IgE-mediated PEG hypersensitivity cannot explain all of the anaphylaxis (and sudden deaths) observed after mRNA vaccine injections.
The CDC has shown they are already aware of hypersensitivity to PEG and its cross-reactivity with polysorbates but are inexplicably not recommending large-scale pre-screening to prevent harm to those who might be hypersensitive to PEG/polysorbate and don’t know it yet.
“CDC considers history of the following to be contraindication to vaccination with both the Pfizer-BioNTech and Moderna COVID-19 vaccines. Severe allergic reaction such as anaphylaxis after previous dose of mRNA COVID-19 vaccine or any of its component, immediate allergic reaction of any severity to previous dose of mRNA COVID-19 vaccine, or any of its component including polyethylene glycol (PEG) and immediate allergic reaction of any severity to polysorbate due to the potential cross-reaction hypersensitivity between the vaccine ingredient PEG and polysorbate. Persons with immediate allergic reaction to the first dose of mRNA COVID-19 vaccine should not receive additional doses of either of the mRNA COVID-19 vaccines because the two authorized vaccines contain ingredients in common. I will also mentioned that these persons should not receive mRNA COVID-19 vaccination at this time unless they have been evaluated by an allergist immunologist and determined the person can safely receive the vaccine such as vaccination under observation or settings with advanced medical care available.” [emphasis added]
https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
2. CARPA (Complement Activation Related Pseudoallergy)
A 2019 Review on complement activation related pseudoallergy (CARPA) following exposure to PEGilated liposomes provides insight into a second mechanism that may be responsible for some of the life-threatening reactions being reported.
What is complement? Complement is part of the innate immune system and so it is part of our defense against microbes that enter the bloodstream. In certain circumstances, this complement pathway is over-activated, setting off a complex cascade that gets out of control, causing the release of complement products called anaphylatoxins. This can happen in response to liposomes as well as some other substances.
CARPA is anaphylaxis that’s mediated by complement-derived anaphylatoxins rather than by IgE antibodies. Hence, it can happen to anyone at first exposure to an offending substance (e.g. liposomes, MRI contrast dye, etc.) without a preexisting hypersensitivity. CARPA is an anaphylactic reaction to liposomes themselves (not to PEG per se) but it is enhanced by PEGylation. CARPA has been studied with respect to liposome formulations and the mode of delivery that are used for cancer drugs; however, some aspects may be relatable to PEGylated LNPs in mRNA vaccines to account for the observed excess in the frequency of anaphylaxis (and other sudden death events).
Some liposomal formulations also have the potential to cause circulatory collapse and spontaneous death (when tested in pigs) depending on the cholesterol content of liposomes:
“. . . .pulmonary hypertension induced by liposomes occurs through the activation of classical and alternative pathways of the complement system via antibodies and C3 binding [94]. The presence of cholesterol in LMV at a level <45 mol% did not affect the pulmonary reaction, while >45 mol% cholesterol increased the complement activation in such a degree that a very small amount (5 mg) of these vesicles lead to circulatory collapse with spontaneous death in almost 50% of the pigs [94]. The possible explanation for this difference is that the excess cholesterol in the vesicles would become exposed on the surface of liposome membrane as microcrystal aggregates and therefore become more susceptible to interaction with naturally occurring anti-cholesterol antibodies [94]. Accordingly, it could be concluded that vasoactivity of vesicles is correlated with surface zeta potentials (positive or to a lesser extent negative) and improperly formulated vesicles containing cholesterol levels in excess of what can be accommodated in phospholipid membranes.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6598536/
An article in the journal Cell provides details on how Moderna’s LNPs are made. The molar ratio of cholesterol in their LNP formulations is 38.5%, which is not far below the 45% threshold that was fatal for pigs.
LNP formulations were prepared using a modified procedure of a method previously described.17 Briefly, lipids were dissolved in ethanol at molar ratios of 50:10:38.5:1.5 (ionizable lipid:DSPC:cholesterol:PEG lipid). [emphasis added]
https://www.cell.com/molecular-therapy-family/nucleic-acids/fulltext/S2162-2531(19)30017-4
A batch-to-batch variation in how these lipids are mixed for LNP production or aggregated during storage cannot be excluded as a possibility that might result in “hot lots” that account for the observed fatal or near-fatal clusters of reactions. It should not be overlooked that a human weighs much less than a pig, which could put us at risk of harm from improperly formulated or stored liposomes at a much lower dose than 5 mg reported in the study for pigs. (For comparison, Moderna’s vaccine contains 1.93 mg of lipids per dose). Without a proper forensic investigation into every death following the exposure to these vaccines, we will never know what has happened.
TLR Activation and TNF-α
3. A third mechanism of injury that can lead to death is explained by Sin Hang Lee, MD, in a filing with the FDA:
The FDA should know that all vaccines, including the Pfizer mRNA vaccine, have associated potential adverse reactions after injection into healthy humans. The nanoparticles composed of mRNA coated with phospholipids may act as potent toll-like receptor (TLR) agonists after endocytosis, causing local and systemic surges of IFN-1 and proinflammatory cytokines. The molecular focal point of this reaction is illustrated in the diagram cropped from a review article titled “mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection.” [41]
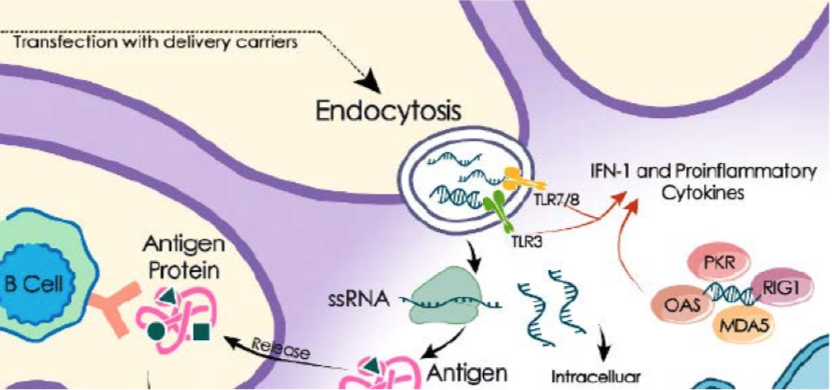
TLR activation may lead to a variety of autoimmune disorders. Some of them are fatal. For example, TLR 7 activation may lead to severe thrombocytopenia in experimental animals, [42] and might have been the cause of death of a 56-year-old physician who developed fatal thrombocytopenia after receiving Pfizer vaccines. [43] Therefore, before introducing such a new vaccine whose active ingredient is synthetic mRNA coated with phospholipids in the form of nanoparticles without safety track record, a high benefit-to-risk ratio must be demonstrated without a reasonable doubt. The FDA is the gatekeeper to maintain this high benefit-to-risk ratio for approval of the Pfizer vaccine. The Petitioner simply requested that the FDA use due diligence to ensure that the preliminary laboratory test results, which the vaccine manufacturer used as the pivotal criteria in qualifying COVID-19 cases as endpoints for vaccine efficacy evaluation, are properly verified. If the vaccine’s efficacy is not as high as claimed due to false-positive test results in the Phase 3 clinical trials, the Petitioner and his fellow citizens may be forced by various business operators to take a vaccine with high risk and uncertain benefits in the name of COVID-19 prevention even if there is no government mandate to do so. . .
The Pfizer vaccine is the first of any prophylactic mRNA vaccines scheduled to be injected into healthy humans without a safety-and-efficacy track record. In principle, the synthetic mRNA encoding the spike protein (S protein) of SARS-CoV-2 is packaged as stable nanoparticles consisting of ionizable cationic lipids, natural phospholipids, cholesterol and polyethylene glycol (PEG). The purpose is to direct the human cells to produce a virus protein as an antigen. If successful, the virus protein produced by the host cells will serve as a subunit virus antigen to stimulate immune responses in the host. However, subunit and synthetic peptide/protein antigens by themselves are relatively weak immunogens and require the assistance of specially designed adjuvants to generate a robust and persistent immune response. The stable nanoparticles composed of ssRNA coated with phospholipids have self-adjuvanting properties after being transfected into the cytoplasm by endocytosis. After entering the endosomal/lysosomal compartments of the cell, these adjuvants can activate certain toll-like receptors to initiate a series of innate immune responses, which are required to boost antibody production. And PEG can extend the half-life of these nanoparticles in the host after injection. Since ssRNAs are potent TLR 7/8 agonists and phospholipids are potent TLR 4 agonists, they will activate a series of toll-like receptors, which will lead to strong and long-lasting adaptive immune responses through tumor necrosis factor-α (TNF-α), interferon-γ (IFN- γ) and other proinflammatory cytokines that are secreted by activated immune cells.[41] However, TNF-α and IFN-γ may cause serious adverse effects in certain genetically and physically predisposed individuals
Since the 1980s, TNF-α, especially in combination with IL-1β, has been known to cause myocardial depression in animals and humans with potentially fatal outcomes. [50-52] Some of the sudden unexpected deaths after injection of the Pfizer vaccine have been reported in the news media. For example, a formerly healthy 41-year-old female healthcare worker died unexpectedly 48 hours after injection of the Pfizer coronavirus vaccine. [53] On January 27, 2021, a local news radio reported that a 60-years old X-ray technician at South Coast Global Medical Center in Santa Ana, California died after receiving 2nd Pfizer vaccine. The deceased apparently died of uncontrollable hypotension and renal failure. [54] These and other similar unexpected deaths after injection of the Pfizer vaccine cannot always be explained away by declaring “lack of evidence linking vaccination to death”. In fact, these unexpected heart failures may be caused by a sudden discharge of TNF-α by macrophages with activated toll-like receptors while these activated cells were clustering in the myocardium. Another vaccine, Gardasil, which is also known to contain viral nucleic acids according to an FDA announcement [55], has been reported to be associated with unexpected deaths among healthy vaccinees after receiving Gardasil injections. At autopsy, these unexpected death cases may show no cause of death. The anatomical findings in the myocardium may range from totally normal to extensive inflammatory cell infiltration. [56, 57]
IFN-γ is known to play important roles in the pathogenesis of autoimmune neuroinflammation, which under certain conditions may lead to multiple sclerosis. [58]
Overproduction of TNF-α in women may lead to obstetric complications, such as recurrent pregnancy loss, early and severe pre-eclampsia, and recurrent implantation failure syndrome. [59]
Therefore, there is scientific evidence in the public domain to suggest that potential health risks may be associated with injection of mRNA vaccines into the human body. The FDA is responsible to ensure that the benefits of the newly introduced mRNA vaccine indeed outweigh its potential risks. [bold emphasis added]
https://childrenshealthdefense.org/wp-content/uploads/Dr.-Lee-Amended-Reply-Final.pdf

