Weekly Stories about What’s Happening in Washington State and Stories of Interest to Washingtonions
by Gerald Braude
Advocating for the COVID-19 Injured in Washington
On the June 3, 2022 “An Informed Life Radio” show on 1150 AM and CHD-TV hosted by Bernadette Pajer and Xavier Figueroa, three representatives from the advocacy group COVID-19 Vaccine Injured of Washington (CVIWA) discussed their various adverse reactions to the COVID-19 shots, such as neurological issues and a mini-stroke. At first glance, the oddest reaction was a thirty-two-year-old’s case of appendicitis two weeks after her first jab. After her emergency surgery, she considered the appendicitis following the jab to be just a coincidence. So, she got the second shot, and, two weeks later, suffered a mini-stroke.
Bernadette said to her, “Appendicitis is reported frequently to VAERS as an adverse reaction, and I don’t blame you for not connecting it. Who would think that appendicitis was a vaccine reaction? That isn’t anything that anybody has ever discussed before. But now, we’re learning that the mRNA particles travel to all the organs, so the reactions are biologically plausible.”
The Vaccine Adverse Event Reaction System (VAERS web site) shows that appendicitis in Washington is a relatively common occurrence.
The May 27, 2022 VAERS data release has the following heading:
Found 19 cases where Location is Washington and Vaccine is COVID19 and Symptom is Appendicitis
The age breakdown is as follows:
Ages 18-29: 2
Ages 30-39: 6
Ages 40-49: 5
Ages 50-59: 1
Ages 60-64: 2
Ages 65-79: 3
The latest case was entered on January 12, 2022 of a 64-year-old woman with the following submitted write-up: Patient received Pfizer COVID vaccine on 5/13/21 and 6/10/21. On 1/3/22 and 1/9/22, patient tested positive for COVID. On 1/9/22, patient admitted to our inpatient facility for acute appendicitis with COVID-19 pneumonia and acute COPD exacerbation. On 1/12/22, patient underwent laparoscopic appendectomy and biopsy of liver nodule. As of today (1/12/22), patient is still admitted in our med/surgical unit.
One of the current aims of COVID-19 Vaccine Injured of Washington is moving compensation of COVID-19 shot injuries from the Countermeasure Injury Compensation Program (CICP) to the Vaccine Injured Compensation Program (VICP). Rachael Ritala from CVIWA said that 8,010 CICP claims have been submitted, but not a single payout has occurred. Furthermore, the reimbursements cover only medical expenses and loss of income. The CICP provides no compensation for pain and suffering, and there is a high burden of proof on the claimants.
Bernadette added that, as a covered countermeasure under the PreP Act and an Emergency Use Authorization (EUA) product, those who make, distribute, require, and administer them are shielded liability and the only option for those who are injured is to apply for compensation from the CICP. COVID-19 shot injuries carry a one year statute of limitations, and the claimant needs to be injured for at least six months. Furthermore, the government will not pay any legal fees to help with the filing.
On the other hand, as Rachael noted, the VICP pays past and future custodial rehabilitation claims without any limits, based on the need for care. VICP also provides up to $250,000 for actual and projected pain and suffering. It can take up to eight years, however, for claims to make it through the VICP process, and most claims are denied.
When asked about strategies for moving COVID-19 shot injuries from the CICP to the VICP, Lindsay Burmeister from COVID-19 Vaccine Injured of Washington said, “We don’t have any legislation drafted at this point, but if there are any attorneys who want to reach out to us who have experience in this, we’d love to work with them. But right now, we’re advocating with members of [the legislature] and letting them know how deficient the CICP is while sharing our experiences.”
The web site for COVID-19 Vaccine Injured of Washington has a link for the CICP on its News/Media page. The site also serves as an alternative forum to the censored social media groups. The Contact section and link at the bottom of its home page is an excellent starting point for connecting with others in WA injured by the COVID-19 shots.
The web address is www.cviwa.org
Covid Vaccine – Covid Vaccine Injured of Washington (covidvaccineinjuredwa.com)
VAERS Removes Another Washington COVID-19 Shot Death
An article in the May 24, 2022 issue of ICWA News and Views showed that as of May 13, 2022, the Vaccine Adverse Event Reaction System (VAERS) on the Center for Disease Control (CDC) web site showed 202 deaths in Washington. The June 3, 2021 VAERS release showed 201 deaths for Washington.
A search on the MedAlerts.org wayback machine shows the deletion with the following heading:
From the 5/13/2022 release of VAERS data (an older release, current is 5/27/2022):
Found 1 cases where Location is Washington and Vaccine is COVID19 and Patient Died and Case was Deleted between the 5/13/2022 and 5/27/2022 releases
The deletion is of an 82-year-old male. His entry date into VAERS was June 29, 2021. The web page gives no date or explanation for the deletion.
The May 24, 2022 ICWA News and Views also mentioned a VAERS deletion of another Washington death. The heading on the VAERS web site read as follows:
Found 1 cases where Location is Washington and Vaccine targets COVID-19 (COVID19) and Patient Died and Case was Deleted between the 4/1/2022 and 4/22/2022 releases
This VAERS ID 1877553 was of an 87-year-old male whose onset began ten days after taking a first Moderna shot on April 20, 2021. His death was listed as August 28, 2021. The entry date into VAERS was November 17, 2021. The web page gave no date of or explanation for the deletion.
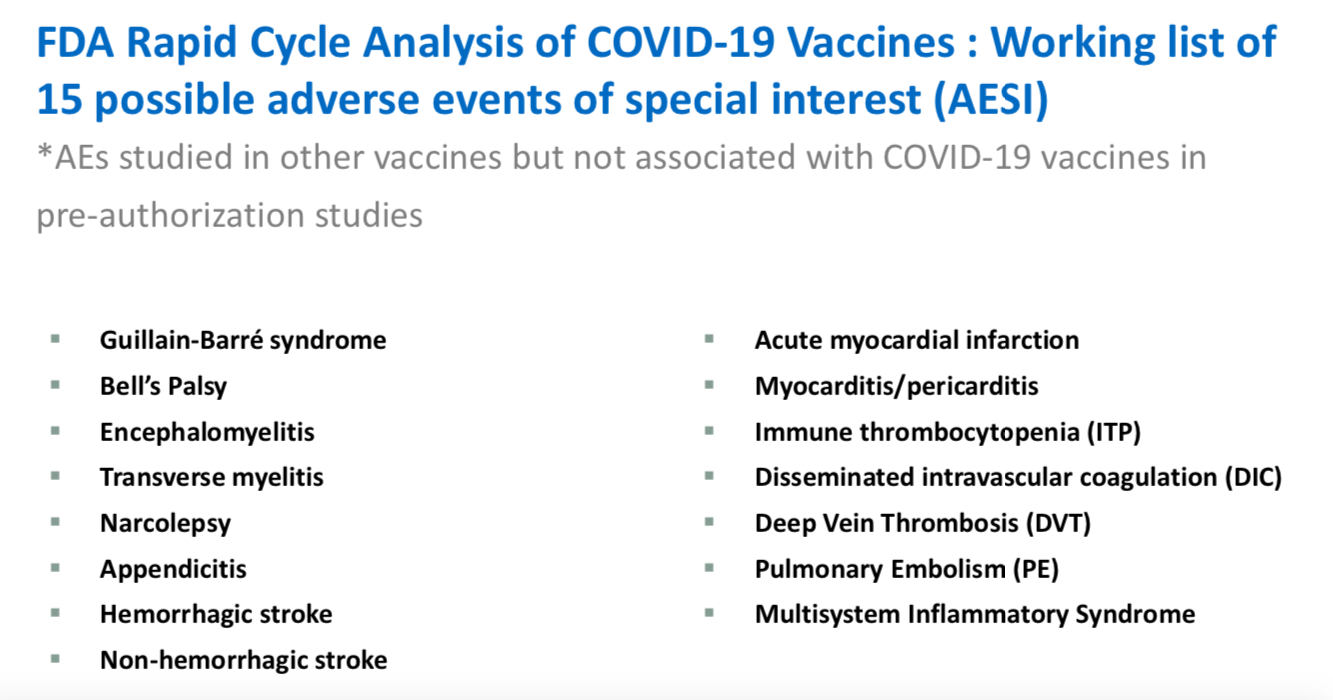
Appendicitis is listed on the FDA’s list of Adverse Events of Special Interest they are watching for, following receipt of a COVID-19 shot.
Note that the release of the Pfizer clinical trial data and whistleblowers are revealing adverse events not presented to the FDA prior to authorization.